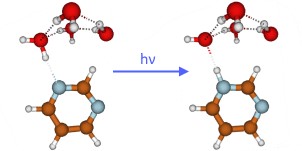
Photoinduced water oxidation in pyrimidine–water clusters: a combined experimental and theoretical study
[Bibtex]
@article{
author = {Xiang Huang, Juan-Pablo Aranguren, Johannes Ehrmaier, Jennifer A. Noble, Weiwei Xie,
Andrzej L. Sobolewski, Claude Dedonder-Lardeux, Christophe Jouvet and Wolfgang
Domcke},
title = {Photoinduced water oxidation in pyrimidine–water clusters: a combined
experimental and theoretical study},
journal = {Physical Chemistry Chemical Physics},
volume = {22},
number = {12502-12514},
abstract = {The photocatalytic oxidation of water with molecular or polymeric N-heterocyclic chromophores is a topic of high current interest in the context of artificial photosynthesis, that is, the conversion of solar energy to clean fuels. Hydrogen-bonded clusters of N-heterocycles with water molecules in a molecular beam are simple model systems for which the basic mechanisms of photochemical water oxidation can be studied under well-defined conditions. In this work, we explored the photoinduced H-atom transfer reaction in pyrimidine�water clusters yielding pyrimidinyl and hydroxyl radicals with laser spectroscopy, mass spectrometry and trajectory-based ab initio molecular dynamics simulations. The oxidation of water by photoexcited pyrimidine is unequivocally confirmed by the detection of the pyrimidinyl radical. The dynamics simulations provide information on the time scales and branching ratios of the reaction. While relaxation to local minima of the S1 potential-energy surface is the dominant reaction channel, the H-atom transfer reaction occurs on ultrafast time scales (faster than about 100 fs) with a branching ratio of a few percent.},
year = {2020},
type= {Article},
}- DOI number: 10.1039/d0cp01562h
- Corresponding authors contact e-mails: domcke@ch.tum.de
The photocatalytic oxidation of water with molecular or polymeric N-heterocyclic chromophores is a topic of high current interest in the context of artificial photosynthesis, that is, the conversion of solar energy to clean fuels. Hydrogen-bonded clusters of N-heterocycles with water molecules in a molecular beam are simple model systems for which the basic mechanisms of photochemical water oxidation can be studied under well-defined conditions. In this work, we explored the photoinduced H-atom transfer reaction in pyrimidine–water clusters yielding pyrimidinyl and hydroxyl radicals with laser spectroscopy, mass spectrometry and trajectory-based ab initio molecular dynamics simulations. The oxidation of water by photoexcited pyrimidine is unequivocally confirmed by the detection of the pyrimidinyl radical. The dynamics simulations provide information on the time scales and branching ratios of the reaction. While relaxation to local minima of the S1 potential-energy surface is the dominant reaction channel, the H-atom transfer reaction occurs on ultrafast time scales (faster than about 100 fs) with a branching ratio of a few percent.