The Role of Ion Chemistry in the Solar Geoengineering Strategies Against Global Warming
[Bibtex]
@article{
author = {Mauro Satta, Antonella Cartoni, Daniele Catone, Mattea Carmen Castrovilli, Paola
Bolognesi, Nicola Zema, and Lorenzo Avaldi},
title = {The Reaction of Sulfur Dioxide Radical
Cation with Hydrogen and its Relevance in Solar Geoengineering Models},
journal = {ChemPhysChem},
volume = {21},
number = {1-12},
abstract = {SO2 has been proposed in solar geoengineering as a precursor of H2SO4 aerosol, a cooling agent active in the stratosphere to contrast climate change. Atmospheric ionization sources can ionize SO2 into excited states of SO2.+, which quickly reacts with trace gases in the stratosphere. In this work we explore the reaction of H2(D2) with SO2.+ excited by tunable synchrotron radiation, leading to HSO2+ + H (DSO2+ + D), where H contributes to O3 depletion and OH formation. Density Functional Theory and Variational Transition State Theory have been used to investigate the dynamics of the title barrierless and exothermic reaction. The present results suggest that solar geoengineering models should test the reactivity of SO2.+ with major trace gases in the stratosphere, such as H2 since this is a relevant channel for the OH formation during the nighttime when there is not OH production by sunlight. OH oxides SO2, triggering the chemical reactions leading to H2SO4 aerosol.},
year = {2020},
type= {Article},
}- DOI number: 10.1002/cphc.202000194
- Copyright Wiley-VCH GmbH. Reproduced with permission.
- Corresponding authors contact e-mails: mauro.satta@cnr.it; antonella.cartoni@uniroma1.it
Global warming is one of main worries for our planet. One of the strategies to mitigate this effect is the solar geoengineering which mimics the natural emission of sulfur dioxide, SO2, by volcanic eruption. This molecule has been proved to reflect sunlight and therefore to reduce global temperatures. Therefore it has been proposed to release in the stratosphere SO2 as a precursor of H2SO4 aerosol, a cooling agent active in the stratosphere to contrast climate change. Theoretical models have been developed to predict the right amount of the cooling agent necessary to produce a climate cooling without a severe irreversible alteration of the global climate. These models however consider only neutral molecules, their thermal reactions and photoinduced dissociation (Figure 1 right panel). These processes occur only in day time when the sunlight, dissociating the ozone molecules, leads to the formation of OH which triggers the SO2 oxidation to H2SO4 aerosol. Actually, clear evidences exist that a significant amount of SO2 is oxidized during the night. Thus we propose that, reactions involving the radical cation SO2.+, which can be produced in the stratosphere by ionizing radiation (cosmic rays and space events), in both day and night times should contribute to the production of the H2SO4 aerosol. The low ion/neutral density ratio of about 10-12 can be counterbalanced by the fact that ion-molecule reactions are generally much faster than the neutral-neutral ones (i. e. the neutral chemistry involved in the conversion of SO2 into H2SO4 takes approximately 30 days). Atmospheric ionization sources can ionize SO2 into excited states of SO2.+, which quickly reacts with trace gases in the stratosphere such as H2 (about 0.5 ppmv) and H2O (about 3-4 ppm) and produce the atmospheric relevant oxidant OH (Figure 1, left panel).
A combined experimental and theoretical study of the reaction dynamics of hot SO2.+ ions, prepared by tunable synchrotron radiation, and rigid neutral species with high vibrational frequencies as H2 has been performed. The results of this work and the ones of the previous one (Cartoni et al. Chem. Eur. J. 23 (2017) 6772-6780), where the reaction of SO2 ions with H2O has been studied, prove that the SO2 ion chemistry should not be neglected in the atmospheric models for solar geoengineering strategies.
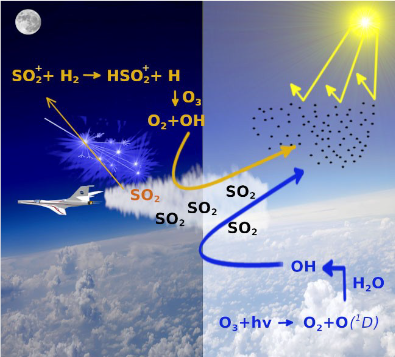
TOC from Satta et al. ChemPhysChem, 2020, 21, 1-12; doi.org/10.1002/cphc.202000194. Copyright Wiley-VCH GmbH. Reproduced with permission.